Miha Jerala, Matic Bošnjak, Stanislav Gobec, Barbara Breznik, Janko Kos and Milica Perišić Nanut
Abstract
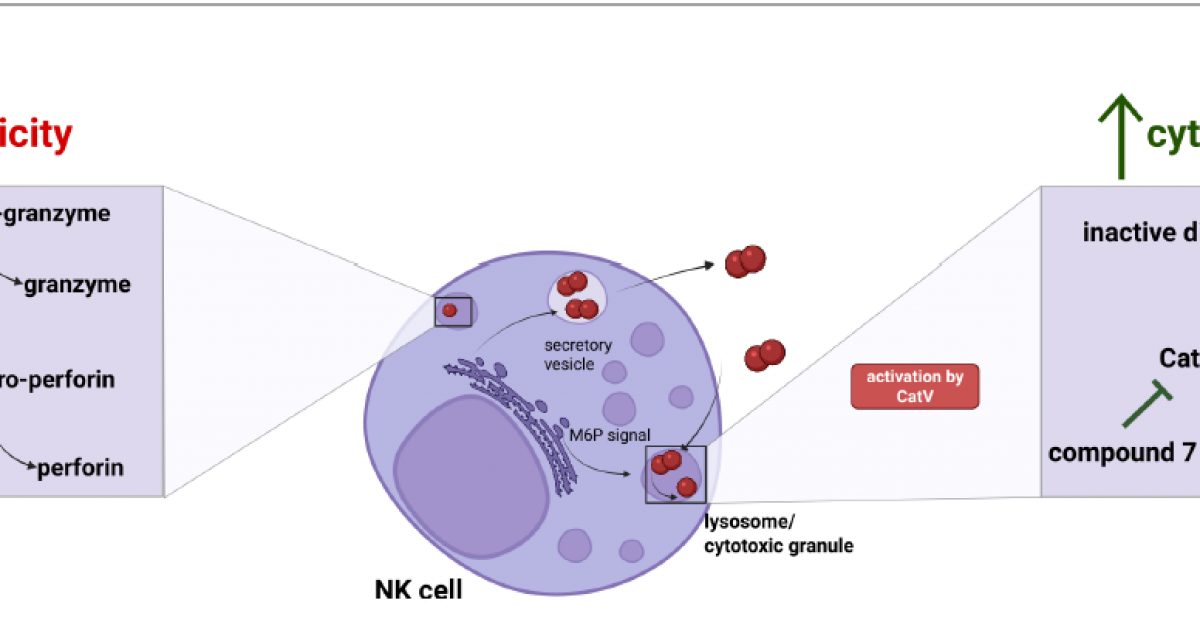
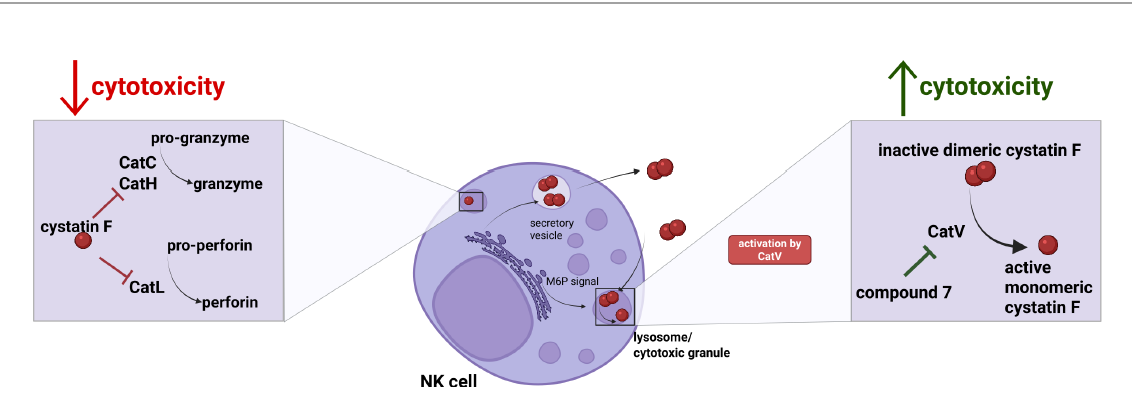
Introduction: Glioblastoma (GBM) is a highly invasive brain tumor with limited treatment options and poor prognosis. Natural killer (NK) cells are key effectors of antitumor immunity, capable of eliminating cancer stem-like cells. However, GBM creates an immunosuppressive microenvironment that limits NK cell function. Here, we identify cystatin F as an immunosuppressive factor involved in regulating NK cell granule-mediated cytotoxicity.
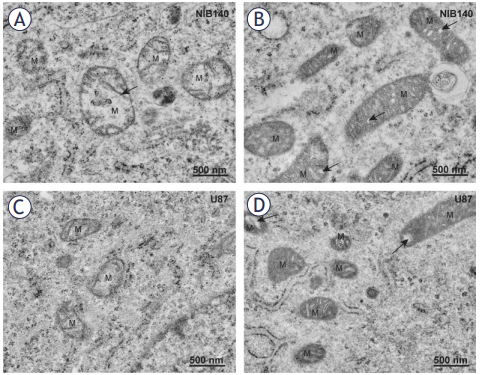
Methods: We analyzed cystatin F expression in GBM and its correlation with immune exhaustion markers. NK cell activity was compared between GBM patients and healthy donors. In vitro co-cultures of cystatin F-expressing microglial cells and glioblastoma stem-like cells were used to assess NK cell function. To block cystatin F activation from dimeric to active monomeric form, a small-molecule inhibitor of cathepsin V, the activating protease, was applied.
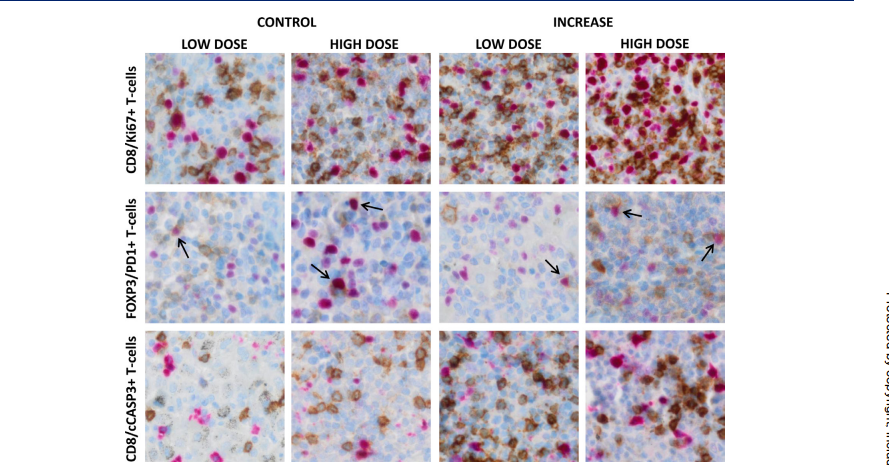
Results: Cystatin F expression correlated with immune exhaustion and suppression markers in GBM. NK cells from patients showed reduced cytotoxicity compared to healthy donors. Co-cultures confirmed that cystatin F-expressing microglia impaired NK cell cytotoxicity, while inhibition of cathepsin V restored NK cell function in standard cytotoxicity assays, 3D spheroids, and microfluidic perfused models.
Discussion: These results indicate that cystatin F mediates NK cell suppression in GBM. Targeting its activation enhances NK cell cytotoxicity, offering a potential strategy to improve NK-based immunotherapy for glioblastoma.